Build App Faster With
Kissflow's Pre-Built Low-Code Apps
Building enterprise-grade low-code applications with Kissflow requires minimal ramp-up time, enabling IT teams and business units to collaborate without bottlenecks. Kissflow’s visual development environment empowers process owners to rapidly design and deploy both simple and mission-critical applications—while IT retains full governance and security oversight.
Enterprises can also deploy fully functional, pre-built low-code apps to accelerate modernization, automate complex workflows, and standardize operations across departments. With reusable components, built-in compliance controls, and seamless integration capabilities, Kissflow helps organizations streamline internal processes at scale—without rebuilding systems from scratch.

Deviation Management
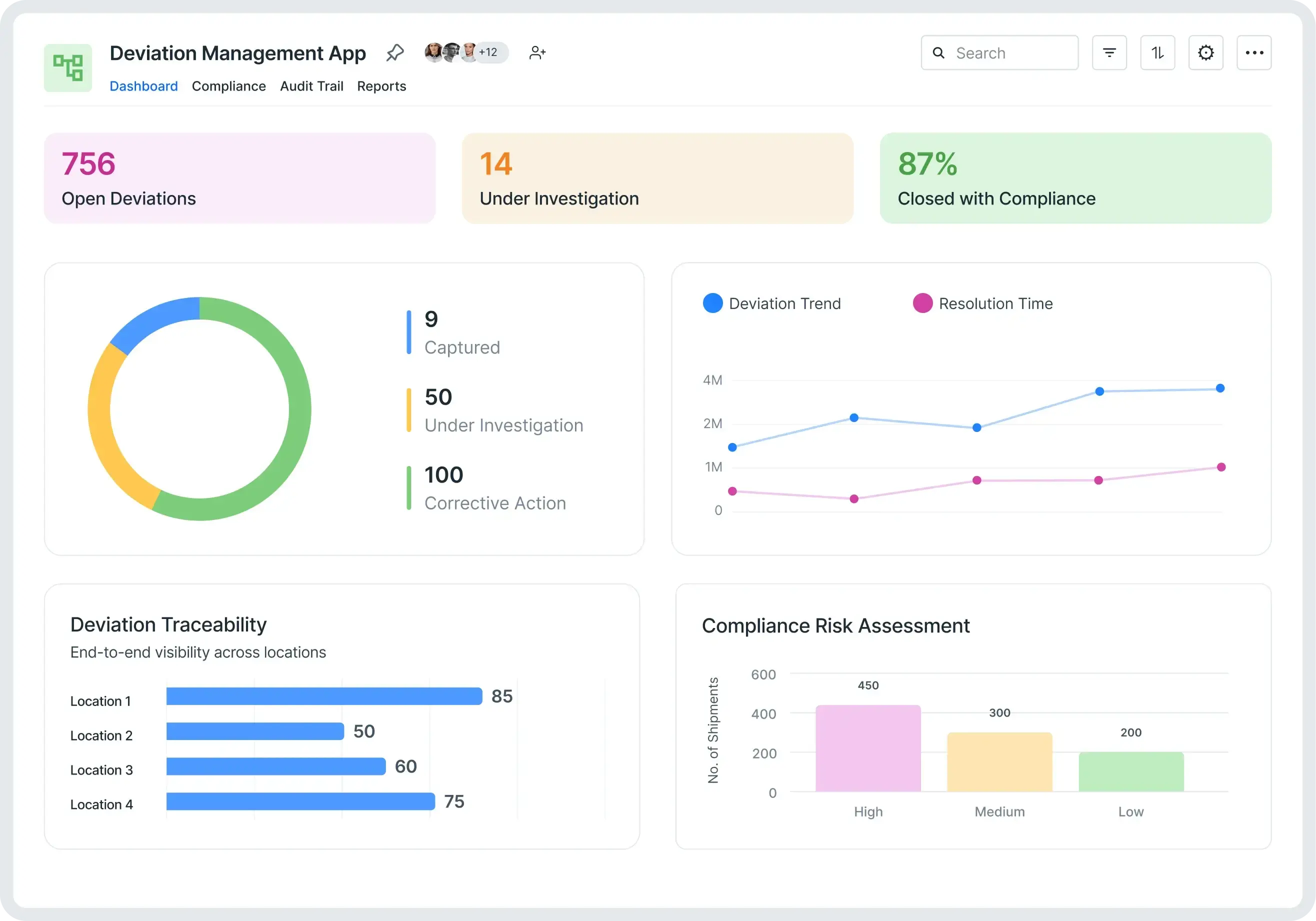
Eliminate compliance gaps and ensure every deviation is managed, documented, and resolved fast. Kissflow’s Deviation Management App is your all-in-one solution to capture, investigate, and close process or quality deviations with full traceability. Automate workflows, standardize corrective actions, and stay audit-ready, whether you’re in manufacturing, pharma, retail, or any process-driven industry.
Creator
Language
English
Category
FMCG
Trusted by regulated industries and quality-driven organizations worldwide



What Is a Deviation Management App?
A Deviation Management App is a digital platform designed to systematically record, review, investigate, and resolve deviations from standard processes or quality norms. Instead of relying on emails, spreadsheets, or disconnected systems, teams gain a centralized, auditable workflow to handle deviations from detection through closure.
It ensures:
-
Every deviation is captured and assigned for investigation
-
Corrective and preventive actions (CAPA) are enforced and tracked
-
Full audit trails for all deviation records and actions
-
Compliance with industry standards (GMP, ISO, FDA, etc.)
Why Do Businesses Need Deviation Management?
Manual deviation tracking often leads to:
-
Missed deviations or incomplete investigations
-
Delayed or forgotten corrective actions
-
Lack of root cause analysis and recurring problems
-
Poor audit readiness and compliance risk
A dedicated app helps streamline these workflows, ensuring every deviation is managed efficiently, with nothing slipping through the cracks.
Key Benefits:
-
Boost compliance by automating deviation capture and closure
-
Enable timely corrective and preventive action with automated notifications
-
Standardize deviation handling across departments and sites
-
Maintain full traceability and documentation for audits and inspections
Modules
-
Deviation Reporting:
Capture deviations via web or mobile forms, including all critical data (date, type, location, involved parties, description, impact). -
Investigation Workflow:
Assign investigations, set due dates, document findings, attach evidence, and escalate if needed. -
CAPA (Corrective & Preventive Action) Tracker:
Enforce action plans, document steps taken, and monitor completion with automated reminders. -
Deviation Review & Approval:
Route for QA/Compliance review, multi-level approvals, and closure with digital signatures. -
Deviation Repository:
Maintain a searchable, exportable database of all deviations for instant audit access. -
Dashboards & Reports:
Visualize trends, root cause categories, overdue actions, and compliance metrics in real time.
Features
-
Deviation submission and assignment
-
Automated investigation and CAPA workflow
-
Root cause analysis tools
-
Multi-level review and approval
-
Audit-ready deviation logs and reports
-
Role-based access and permissions
-
Automated reminders and escalations
-
Compliance documentation exports
-
Dashboards for deviation trends and KPIs
-
Integration with ERP, QMS, and compliance systems
-
Low-code customization for industry-specific needs
-
Click the Enquire button on the app store page.
-
Fill in your innovation process details and team size.
-
Click Submit.
Kissflow team will help you tailor the app to your unique innovation journey.
See how organizations eliminate compliance gaps and audit risks with Kissflow Deviation Management.

This is so easy, even my mom could do this. It was extremely intuitive and straightforward. The watermark was, 'I don't need to call IT to do this. I can do it myself.
Renee Villarreal
Senior IT Manager
Industry
Energy
HeadQuaters
USA
Key Highlights
450+
Process
10x
ROI
10,000+
Users


The beauty of Kissflow is how quick and easy it is to create the apps I need. It's so user-friendly that I made exactly what I needed in 30 minutes.
Oliver Umehara
IT Manager
Industry
Telecom & Media
HeadQuaters
Japan
Key Highlights
28+
Processes
42
Group Companies
70+
Users


We seek to go beyond incremental efforts not only in sustainability but also in everything we do. With Kissflow, FPH and its subsidiaries were able to digitize dramatically major operations, especially in their finance and accounts operations.
Joseph Arnel Chavez
Assistant Manager
Industry
Energy & Utilities
HeadQuaters
Philippines
Key Highlights
100+
Office Processes Automated
1,000+
Monthly Paperless Processes
10,000+
Employees
Frequently Asked Questions
Explore other related apps

Innovation Lifecycle Management
By Kissflow
Drive innovation from idea to execution with ease. Kissflows Innovation Lifecycle Management app helps you capture ideas, evaluate them, manage development pipelines, and track ROI all in one place. Built for teams seeking to make innovation systematic and scalable, its the ideal low-code platform for modern enterprises.
Custom Quote

New Product Launch Management
By Kissflow
Launch new products with speed and precision. Kissflows New Product Launch app centralizes all launch activities from planning and approvals to tracking progress and performance so your teams can execute winning launches every time, no matter the complexity.
Custom Quote
Accelerate and scale app development with Kissflow
Customize with pre-built templates
Build custom low-code apps quickly with pre-built templates.
Tackle internal app backlogs
Implement strategies to clear your internal application backlog quickly.
Join enterprises that trust Kissflow
Enterprises use our low-code platform to streamline app development.
Didn’t find what you're looking for?
Let us know what we can build for you